Acceptable Quality Level is a sampling plan according to the batch size and inspection levels. Some buyers have this requirement to follow the AQL, therefore, some manufacturing units have started following this concept in pharmaceuticals.
AQL helps to determine the following things:
2. Acceptance criteria to reject or approve the batch.
The concept of Acceptable Quality Level is taken from ISO 2859-1 – Sampling Procedures for Inspection. This is a statistical tool that helps the buyer in product inspection. Most of the suppliers involved in international trade are aware of it. AQL is used to assure the product quality using international inspection standards. AQL is determined by two tables one for the number of samples to be taken and other for acceptance limits.
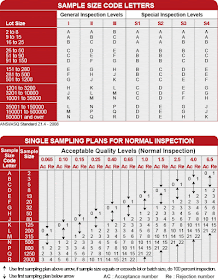
First of all, a code letter is selected from the table 1 according to the batch/lot size. For example, if batch size is 5,00,000 then the letter will be P for the inspection level II. Inspection level is selected as per the criticality of the defect.
Now find the number of samples to be taken using table 2. 800 units should be sampled for the letter P. Acceptable limit for this batch is 21 units for 1.5 and above AQL. If 22 units found defective then batch will be rejected.
Using Acceptable Quality Level in pharmaceuticals can help to meet the international standards for pharmaceutical inspection. A sampling plan can be created for pharmaceutical finished products using these AQL tables.




Very informative article
ReplyDeleteThanks Professor Ankur very much
ReplyDeleteif you add the references as well that would be great
ReplyDelete