Ultraviolet and visible absorption spectrophotometry is the measurement of the absorption of monochromatic radiation by solutions of chemical substances, in the range of 185 nm to 380 nm, and 380 nm to 780 nm of the spectrum, respectively.
The magnitude of the absorption of a solution is expressed in terms of the absorbance, A, defined as the logarithm to base 10 of the reciprocal of transmittance (T) for monochromatic radiation:
where 10 is the intensity of the incident radiation I is the intensity of the transmitted radiation. The absorbance depends on the concentration of the absorbing substance in the solution and the thickness of the absorbing layer taken for measurement.
It is evaluated by the expression
A(l percent, 1 cm) =A/cl,
where c is the concentration of the absorbing substance expressed as percentage w/v and I is the thickness of the absorbing layer in cm. The value of A (1 percent, 1 cm) at a particular wavelength in a given solvent is a property of the absorbing substance.
 Use solutions of potassium dichromate UV which has been previously dried to constant weight at 130°. For the control of absorbance at 235 nm, 257 nm, 313 nm and 350 nm, dissolve 57.0-63.0 mg of potassium dichromate UV in 0.005 M sulphuric acid and dilute to 1000.0 ml with the same acid. For the control of absorbance at 430 nm, dissolve 57.0-63.0 mg of potassium dichromate UV in 0.005 M sulphuric acid and dilute to 100.0 ml with the same acid.
Use solutions of potassium dichromate UV which has been previously dried to constant weight at 130°. For the control of absorbance at 235 nm, 257 nm, 313 nm and 350 nm, dissolve 57.0-63.0 mg of potassium dichromate UV in 0.005 M sulphuric acid and dilute to 1000.0 ml with the same acid. For the control of absorbance at 430 nm, dissolve 57.0-63.0 mg of potassium dichromate UV in 0.005 M sulphuric acid and dilute to 100.0 ml with the same acid.
A statement in an assay or test of the wavelength at which maximum absorption occurs implies that the maximum occurs either precisely at or within ± 2 nm of the given wavelength.
Likewise, a statement in a test of the absorbance, A, at a given wavelength or at the maximum at about a specified wavelength implies that the measured absorbance is within ± 3 per cent of the stated value.
When an assay or test prescribes the use of a Reference Substance, make the spectrophotometric measurements with the solution prepared from the Reference Substance by the official directions and then with the corresponding solution, prepared from the substance under examination. Carry out the second measurement as quickly as possible after the first, using the same cell and same experimental conditions.
Unless otherwise specified, the requirements in the monographs for light absorption in the tests and assays apply to the dried or anhydrous material, where a standard is given for loss on drying of the content of water, respectively. Similar considerations apply where standards are given for solvent content. In calculating the result, the loss on drying or contents of water or solvent, determined by the method specified in the monograph, are taken into account.
A first order-derivative spectrum is a plot of the gradient of the absorption curve (rate of change of the absorbance with wavelength, dA/dA ) against wavelength.
A second order-derivative spectrum is a plot of the curvature of the absorption spectrum (d²A/dλ²) against wavelength. If the absorbance follows the Beer- Lambert relationship, the second order-derivative at any wavelength A is related to the concentration by the following expression.
d²A/dλ²= d²A (1 percent, 1 cm) x cd/dλ²
where, A = the absorbance at wavelength A,
A (1 per cent, 1 cm) = the specific absorbance at wavelength λ
c = the concentration of the absorbing solute express as a percentage w/v,
d = the thickness of the absorbing layer in em.
The magnitude of the absorption of a solution is expressed in terms of the absorbance, A, defined as the logarithm to base 10 of the reciprocal of transmittance (T) for monochromatic radiation:
where 10 is the intensity of the incident radiation I is the intensity of the transmitted radiation. The absorbance depends on the concentration of the absorbing substance in the solution and the thickness of the absorbing layer taken for measurement.
It is evaluated by the expression
A(l percent, 1 cm) =A/cl,
where c is the concentration of the absorbing substance expressed as percentage w/v and I is the thickness of the absorbing layer in cm. The value of A (1 percent, 1 cm) at a particular wavelength in a given solvent is a property of the absorbing substance.
Unless otherwise stated, measure the absorbance at the prescribed wavelength using a path length of 1 cm and at 24° to 26°. Unless otherwise stated, the measurements are carried out with reference to the same solvent or the same mixture of solvents.
The two empty cells used for the solutions under examination and the reference liquid must have the same spectral characteristics. Where double-beam-recording instruments are used, the solvent cell is placed in the reference beam.
Apparatus
A spectrophotometer, suitable for measuring in the ultraviolet and visible ranges of the spectrum consists of an optical system capable of producing monochromatic light in the range of 200 nm to 800 nm and a device suitable for measuring the absorbance.The two empty cells used for the solutions under examination and the reference liquid must have the same spectral characteristics. Where double-beam-recording instruments are used, the solvent cell is placed in the reference beam.

Control of wavelengths
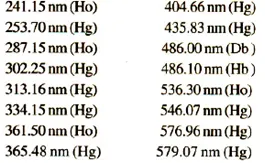
Verify the wavelength scale using the absorption maxima of holmium perchlorate solution, the line of hydrogen or deuterium discharge lamp or the lines of a mercury vapor are shown below. The permitted tolerance is ± 1nm for the range 200 nm to 400 nm and ± 3 nm for the range 400 nm to 600 nmControl of absorbance
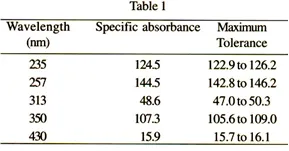
Check the absorbance using suitable filters or a solution of potassium dichromate UV at the wavelengths indicated in Table 1, which gives for each wavelength the exact values and permitted limits of the specific absorbance. The tolerance for the absorbance is ± 0.01. Use solutions of potassium dichromate UV which has been previously dried to constant weight at 130°. For the control of absorbance at 235 nm, 257 nm, 313 nm and 350 nm, dissolve 57.0-63.0 mg of potassium dichromate UV in 0.005 M sulphuric acid and dilute to 1000.0 ml with the same acid. For the control of absorbance at 430 nm, dissolve 57.0-63.0 mg of potassium dichromate UV in 0.005 M sulphuric acid and dilute to 100.0 ml with the same acid.
Use solutions of potassium dichromate UV which has been previously dried to constant weight at 130°. For the control of absorbance at 235 nm, 257 nm, 313 nm and 350 nm, dissolve 57.0-63.0 mg of potassium dichromate UV in 0.005 M sulphuric acid and dilute to 1000.0 ml with the same acid. For the control of absorbance at 430 nm, dissolve 57.0-63.0 mg of potassium dichromate UV in 0.005 M sulphuric acid and dilute to 100.0 ml with the same acid.Limit of stray light
Stray light may be detected at a given wavelength with suitable filters or solutions; for example, the absorbance of a 1.2 percent w/v solution of potassium chloride in a 1cm cell should be greater than 2.0 at about 200 nm when compared with water as reference liquid.Resolution power
When stated in a monograph, record the spectrum of 0.02 percent v/v solution of toluene in hexane UV. The ratio of the absorbance at the minimum at about 269 nm to that at the maximum at about 266 nm is not less than 1.5 unless otherwise specified in the monograph.Spectral slit width
When measuring the absorbance at an absorption maximum the spectral slit width must be small compared with the half width of the absorption band otherwise erroneously low absorbance will be measured. Particular care is needed for certain substances and the instrumental slit width used should always be such that further reduction does not result in an increased absorbance reading.Cells
The absorbances of the cells intended to contain the solution under examination and the reference liquid, when filled with the same solvent should be identical. If this is not the case, an appropriate correction must be applied. The tolerance on the path length of the cells used is ± 0.005 cm. Cells should be cleaned and handled with great care.Solvents
In measuring the absorbance of a solution at a given wavelength, the absorbance of the reference cell and its contents should not exceed 0.4 and should preferably be less than 0.2 when measured the reference to air at the same wavelength. The solvent in the reference cell should be of the same lot as that used to prepare the solution and must be free from fluorescence at the wavelength of measurement. Ethanol (95 percent), ethanol, methanol, and cyclohexane, used as solvents, should have an absorbance measured in a 1 cm cell at about 254 nm with reference to water not exceeding 0.10.Determination of absorbance
Unless otherwise directed, measure the absorbance at the prescribed wavelength using a path length of 1 cm at 24° to 26° If necessary, the path length may be varied provided that compliance with Beer's Law has been shown over the range in question.A statement in an assay or test of the wavelength at which maximum absorption occurs implies that the maximum occurs either precisely at or within ± 2 nm of the given wavelength.
Likewise, a statement in a test of the absorbance, A, at a given wavelength or at the maximum at about a specified wavelength implies that the measured absorbance is within ± 3 per cent of the stated value.
When an assay or test prescribes the use of a Reference Substance, make the spectrophotometric measurements with the solution prepared from the Reference Substance by the official directions and then with the corresponding solution, prepared from the substance under examination. Carry out the second measurement as quickly as possible after the first, using the same cell and same experimental conditions.
Unless otherwise specified, the requirements in the monographs for light absorption in the tests and assays apply to the dried or anhydrous material, where a standard is given for loss on drying of the content of water, respectively. Similar considerations apply where standards are given for solvent content. In calculating the result, the loss on drying or contents of water or solvent, determined by the method specified in the monograph, are taken into account.
Derivative spectrophotometry
Derivative spectrophotometry involves the transformation of absorption spectra (zero-order) into first -, second- or higher order derivative spectra.A first order-derivative spectrum is a plot of the gradient of the absorption curve (rate of change of the absorbance with wavelength, dA/dA ) against wavelength.
A second order-derivative spectrum is a plot of the curvature of the absorption spectrum (d²A/dλ²) against wavelength. If the absorbance follows the Beer- Lambert relationship, the second order-derivative at any wavelength A is related to the concentration by the following expression.
d²A/dλ²= d²A (1 percent, 1 cm) x cd/dλ²
where, A = the absorbance at wavelength A,
A (1 per cent, 1 cm) = the specific absorbance at wavelength λ
c = the concentration of the absorbing solute express as a percentage w/v,
d = the thickness of the absorbing layer in em.


No comments:
Post a Comment
Please don't spam. Comments having links would not be published.