1.0 Objective
This document describes the testing procedure for the efficacy of different types of chemical disinfectants.
2.0 Scope
This document provides the procedure for validating the sanitizers and the sanitization procedure being followed in the manufacturing and the testing facilities in pharmaceuticals.
3.0 Reference Document
SOP for microbiological culture media preparationDisinfectant Efficacy Test
4.0 Pre-Requisites
Prior to conducting/executing the General validation protocol following things must be available
4.1 Microbial Standard Cultures
4.1.1 Bacillus subtilis
4.1.2 Escherichia coli
4.1.3 Staphylococcus aureus
4.1.4 Candida albicans
4.1.5 Aspergillus niger
4.1.6 Environment isolates
4.2 Disinfectants: All disinfectants used in the facility
4.3 Micropipettes
4.4 Sterilized tips and Petri plates
5.0 Responsibility
Responsibilities of Quality Assurance and Quality control Microbiology personnel involved in activities related to the General validation protocol are defined below
5.1 Quality Assurance (Validation)
5.1.1 Approval of protocol
5.2 Quality Control
5.2.1 Preparation of protocol
5.2.2 Review of protocol
5.2.3 Execution of protocol
6.0 Validation Method
A) Suspension method (Validation of sanitizer)
B) Surface spray/immersion or wipe method (Validation of Sanitization method)
6.1 Suspension Method
6.1.1 Objective:
6.2.1 Objective:
9.1 Test for Suspension method
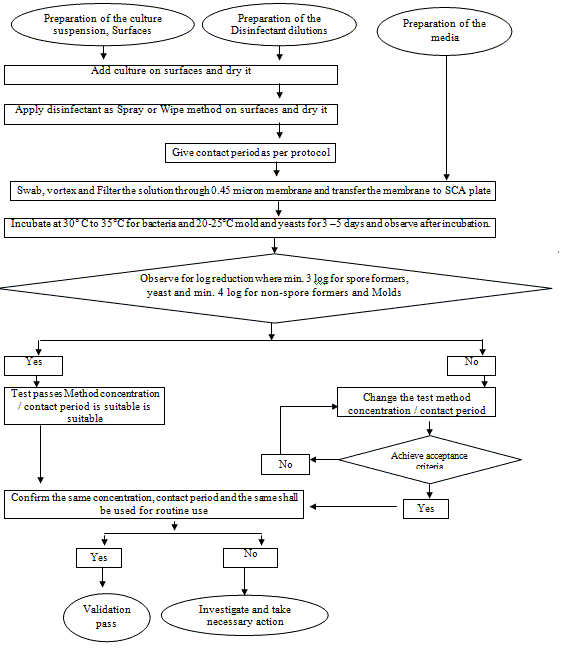
9.2 Test for Surface spray / Wipe method

10.0 Validation Matrix
11.0 Abbreviations
To establish the test concentration and the contact time a suspension test is generally applied. The suspension test estimate the in vitro bactericidal activity of the disinfectant under precise experimental conditions including
• Microbial strain
• Volume of inoculum vs. Disinfectant
• Temperature
• Disinfectant concentration and contact period
• Interfering substances (i.e. inorganic, organic matter)
6.1.2 Procedure:
6.1.2.1 Prepare the culture suspension from the original culture as per the SOP for preparation of Microbiological culture media.
6.1.2.2 Select the dilution which will yield 105 to 106 cells per ml.
6.1.2.3 Prepare 10 ml of test dilution to be tested with sterile distilled water.
6.1.2.4 Vortex the tube for 1 minute.
6.1.2.5 Add 0.1 ml of any one culture containing 105 to 106 cells per ml into the test disinfectant with the decided concentration.
6.1.2.6 The final concentration shall be 104 to 105 cells per the tube. Consider the preparation for 0 min.
6.1.2.7 Prepare like the same above for other 3 different sets for 3 different time intervals.
6.1.2.8 Give a contact time of 0 min, 5 min, 10 min and 15 min.
6.1.2.9 After the specified contact time, filter the samples through a 0.45 m membrane filter.
6.1.2.10 Give three washing of 100 ml each with 0.1 % sterile peptone water/ Sterile Water.
6.1.2.11 After filtration with the help of a sterile forceps take the membrane filter and place it on a Soybean casein digest agar.
6.1.2.12 Incubate the bacterial culture at 30-35 °C for 24 to 48 hours and fungal cultures at 20-25 °C for 72 to 120 hours.
6.1.2.13 After incubation count the number of colonies present on the membrane.
6.1.2.14 Note down the number of colonies.
6.1.2.15 This will be the Observed count after the exposure.
6.1.2.16 Select the plates, which have least to Nil counts.
6.1.2.17 Proceed in the same manner taking all the cultures to be tested.
6.1.2.18 Contact time for the usage of the disinfectant will be set on the basis of the results, which will have least counts.
6.2.1 Objective:
To establish the effectiveness of the test concentration and the contact time generally applied. The suspension test estimates the in vitro bactericidal activity of the disinfectant under precise experimental conditions including.
• Microbial strain
• Preparation of inoculum
• Volume of inoculum vs. Disinfectant
• Temperature
• Disinfectant concentration and contact period
• Interfering substances (i.e. inorganic, organic matter)
With the Spray/immersion or wipe method the following surfaces shall be taken for validation.
Stainless steel (SS)
• Epoxy
• Panel
• PU Paint wall (Poly Urethane Paint)
6.2.2 Procedure:
6.2.2.1 Prepare the culture suspension from the original culture as per the SOP for preparation of Microbiological culture media.
6.2.2.2 Select the dilution which will yield 104 to 105 cells per ml.
6.2.2.3 Take plate of different surfaces such as SS, Epoxy, Panel, PU Paint wall present in the clean room having a surface area of 25 cm2
6.2.2.4 From the previously determined suspension having 104 to 105 cells per ml inoculate one culture on different surfaces mentioned above.
6.2.2.5 With the help of a sterile spatula spread the culture on the surface.
6.2.2.6 Keep it on the LAF bench for drying.
6.2.2.7 After the exposed duration for drying (a) spray the disinfectant (b) disinfect the surface by wipe method.
6.2.2.8 Give a contact time of 0 min, 5 min, 10 min and 15 min.
6.2.2.10 With the help of a sterile moistened swab, swab the surface gently covering all the area of the surface.
6.2.2.11 Place the swab sticks in a test tube having a sterile saline solution and do not hold the swab more than 24 hours.
6.2.2.12 Vortex the test tube gently for 5.0 minutes.
6.2.2.13 Aseptically filter the samples through a 0.45 m membrane.
6.2.2.14 Give three washing of 100 ml each with 0.1 % sterile peptone water / Sterile water.
6.2.2.15 After the filtration with the help of a sterile forceps take the membrane and place it on a Soybean casein digest agar.
6.2.2.16 Incubate the bacterial culture at 30-35 °C for 24 to 48 hours and fungal cultures at 20-25 °C for 72 to 120 hours.
6.2.2.17 After incubation count the number of colonies present on the membrane.
6.2.2.18 Proceed in the same manner taking all the cultures to be tested with the all mentioned disinfectants.
7.0 Acceptance criteria
7.1 The decrease in the bacterial load to the exposed disinfectant indicates that the disinfectant is capable of reducing the contaminant when used in the area. That shall be minimum of 4-log reduction for non-spore forming microorganisms, Yeast and minimum of 3-log reduction shall achieve for Spore-forming organisms, molds with the decided concentration.
7.2 Determine the contact period where the above said population log reduction of microorganisms achieved.
8.0 Observations
8.1 Record the observation of Suspension method in Annexure –I
8.2 Record the observation of Surface spray / Wipe method in Annexure –II
9.1 Test for Suspension method

9.2 Test for Surface spray / Wipe method

Following test matrix is prepared for the initial analytical method validation and revalidation criteria for Efficacy of disinfectants.
Sr. No.
|
Test Description
|
Initial Validation |
Revalidation |
1
|
Suspension Method
|
All disinfectants used
|
Every new disinfectant
|
Change in Disinfectant Concentration
|
|||
Change in Contact time
|
|||
2
|
Surface Spray / Wipe Method
|
All disinfectants used
|
Every new disinfectant
|
Change in Disinfectant Concentration
|
|||
Change in Contact time
|
SOP: Standard Operating Procedure
° C: Degrees centigrade
% : Percentage
CFU: Colony forming units
SCA: Soybean casein digest agar
m: Micron
PU: Poly Urethane
Annexure –I
Results: Suspension Method
Name of the Disinfectant:
|
|||||
Log reduction observed with Contact period (in Min.)
|
|||||
Name of the organism
|
0
|
5
|
10
|
15
|
Significant log reduction observed at
|
Bacillus subtilis
|
|||||
Escherichia coli
|
|||||
Staphylococcus aureus
|
|||||
Candida albicans
|
|||||
Aspergillus niger
|
|||||
Environmental isolate
|
|||||
Annexure –II
Results: Surface Spray / Wipe Method
Surface Selected: Name of the Disinfectant:
|
|||||
Name of the organism
|
Log reduction observed with Contact period (in Min.)
|
||||
0
|
5
|
10
|
15
|
Significant log reduction observed at
|
|
Bacillus subtilis
|
|||||
Escherichia coli
|
|||||
Staphylococcus aureus
|
|||||
Candida albicans
|
|||||
Aspergillus niger
|
|||||
Environmental isolate
|
|||||
Get ready to use editable Validation Protocols in MS-Word FormatView List



how to download this??
ReplyDelete