Stereoisomerism that involves rotational interconversion is known as conformational stereoisomerism. Conformational isomers are those that can be transformed. Single bond rotation is inhibited by rotational energy. If one conformer is to become another, this barrier must be overcome. Conformational Isomerism can only occur at low energy barriers. It comes in a variety of forms. Ethane and Butane are two examples.

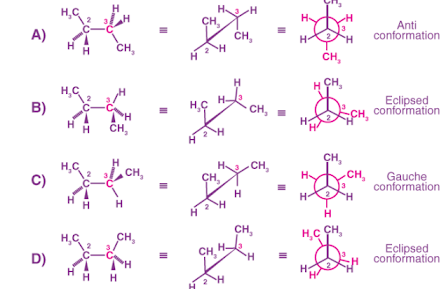
The hydrogen butane molecule comprises the complexity of conformations dependent on its constitution as compared to ethane. Below is a representation of the various forms of butane. Based on the change in potential energy, the C2-C3 bond rotates about the C2-C3. Refrigerants can be made with pure butane. The butane torch makes use of it. Butane usually blends with gasoline. Cordless hair irons are powered by butane cartridges.

Conformational Isomerism
The rotation of atoms about C-C bond axis results in different arrangements of the atoms in space called conformation. Defining a configuration as a set of conformations that are readily interconvertible and thus inseparable.Conformations of Ethane
Rotation of ethane is barred by a barrier of 12 kJ/mol (2.9 kcal/mol). The conformation with the most stability (low energy) is one in which all of the six C–H bonds are separated from each other as far as possible (seemed staggered when viewed end-on). Carbon–hydrogen bonds are closest to one another when they are worn away with a Newman projection (least stable conformation). Every other conformation lies between these two limits. There are three bond-eclipsing interactions between C and H, so we can assign each interaction a value of about 4.0 kJ/mol (1.0 kcal/mol). A mol of propane has a corresponding energy of 14 kJ (3.4 kcal). Conformational isomers can be represented in two ways. Carbon–carbon bonds are depicted as sawhorse representations by angling their orientation in space. A Newman projection shows a circle representing the two carbon atoms and the bond between them. On the left are the bonds associated with the front carbon, and on the right are the bonds associated with the back carbon. The Newman projections have the advantages that they can easily be drawn and that they clearly illustrate the relationships between the different substituents on the carbon atoms.Conformation of n-butane
This organic compound is composed of 4 carbon atoms and is an alkane. The number of carbon atoms in an alkane can be said to be three. The gas Butane is at atmospheric pressure. They are highly flammable liquefied gases.The hydrogen butane molecule comprises the complexity of conformations dependent on its constitution as compared to ethane. Below is a representation of the various forms of butane. Based on the change in potential energy, the C2-C3 bond rotates about the C2-C3. Refrigerants can be made with pure butane. The butane torch makes use of it. Butane usually blends with gasoline. Cordless hair irons are powered by butane cartridges.
Conformation of Cyclohexane
The 3D conformation of Cyclohexane relieves all strain since it is not planar. The chair conformation is its most stable configuration. There is little activation barrier between cyclohexane's different chair conformations: 45 kJ/mol is required for the interconversion or flip of the chair.
Get subject wise printable pdf documentsView Here



No comments:
Post a Comment
Please don't spam. Comments having links would not be published.