The conductivity of a solution (K) is the reciprocal of resistivity (p) which is defined as the quotient of the electric field and the density of the current (flowing in the conducting solution).
The resistance R (in Q) of a conductor of cross-section S (in cm2) and length L (in cm) is given by the expression
R = P x LIS or 11K x LIS; thus, K = l/R x LIS where, LIS corresponds to the ideal cell constant.
The unit of conductivity in the International System is the siemens per metre (S m-1).What is generally used in expressing the electrical conductivity of a solution is siemens per centimetre (S cm-1) or microsiemens per centimetre (~ cm-1).
The resistivity of a solution is expressed in ohm-centimetres (Qcm). Unless otherwise stated, the reference temperature for the expression of conductivity or resistivity is 25°.
Commonly used conductivity cells have cell constants of the order of 0.1 cm-1, 1 cm-1 and 10 cm-1.
Use a certified reference material (such as a solution of potassium chloride) with a conductivity value near the expected value of the solution under examination. Rinse the cell several times with distilled water and at least twice with the certified reference material used for the determination of the cell constant of the conductivity cell. Measure the resistance of the conductivity cell using the certified reference material at 25 ± 1°.The cell constant is given by the expression
 K = RemX Kern,where, Remis the measured resistance in megaohms and Kernis the conductivity of the certified reference material solution used, in microsiemens per centimetre.
K = RemX Kern,where, Remis the measured resistance in megaohms and Kernis the conductivity of the certified reference material solution used, in microsiemens per centimetre.
NOTE - Other certified reference materials may be used especially for cells having a constant of 0.1 cm -1.
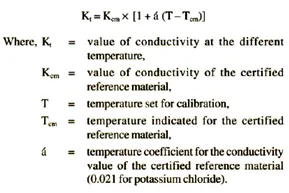
The measured constant K of the conductivity cell must be within 5 percent of the value indicated. If the cell constant is determined at a temperature other than that indicated for the certified reference material, the conductivity value is estimated from the expression:
Related: SOP for Calibration of Conductivity Meter
 After the apparatus has been calibrated with a certified reference material solution, rinse the conductivity cell several times with distilled water and at least twice with the aqueous solution under examination. Carry out successive measurements as described in the individual monograph.
After the apparatus has been calibrated with a certified reference material solution, rinse the conductivity cell several times with distilled water and at least twice with the aqueous solution under examination. Carry out successive measurements as described in the individual monograph.
Water conductivity. A three-stage method of testing is described for Purified Water and Water for Injections (WFI). Testing at the first stage is usually adequate for Purified Water. Testing at two preliminary stages is given for WFI. If the test conditions and conductivity limits are met at either of these preliminary stages, the water meets the requirements of the test. In such cases proceeding to the third stage may not be necessary. Only in the event of failure at the final stage is the sample to be considered as not complying with the requirements of the test.
Stage 1 of the procedure may alternatively be performed online (with suitable modifications of the first step) with instrumentation that has been appropriately calibrated, whose cell constants have been accurately determined, and whose temperature compensation has been disabled. Prior to testing it must be ensured that such instrumentation has been suitably located and fitted in the water system.

2. Using Table 1 find the temperature value that is not greater than the measured temperature and read the corresponding conductivity value that becomes the limit (Do not interpolate).
3. If the measured conductivity is not greater than the table value, the water meets the requirements of the test. If the conductivity is higher than the table value, proceed with testing at stage 2.
5. If the conductivity is not greater than 2.1 ~.cm-1, the water under examination meets the requirements of the test. If the conductivity is greater than 2.1 ~ cm-1, proceed with the testing at stage 3.
7. Using Table 2, determine the conductivity limit at the measured pH value in step 6. If the measured conductivity in step 4 under stage 2 is not greater than the conductivity requirements for the pH determined, the water under examination meets the requirements of the test. If either the measured conductivity is greater than this value or the pH is outside the range of 5.0 to 7.0, the water under examination does not meet the requirements of the test.
Also see: Validation of Purified Water System
The resistance R (in Q) of a conductor of cross-section S (in cm2) and length L (in cm) is given by the expression
R = P x LIS or 11K x LIS; thus, K = l/R x LIS where, LIS corresponds to the ideal cell constant.
The unit of conductivity in the International System is the siemens per metre (S m-1).What is generally used in expressing the electrical conductivity of a solution is siemens per centimetre (S cm-1) or microsiemens per centimetre (~ cm-1).
The resistivity of a solution is expressed in ohm-centimetres (Qcm). Unless otherwise stated, the reference temperature for the expression of conductivity or resistivity is 25°.
Apparatus
The apparatus used is a conductivity meter that measures the resistance of the column of liquid between the belectrodes of the immersed conductivity cell (the measuring device). The meter is supplied with alternating current and is equipped with a temperature probe and a temperature compensation device. The generally used conductivity cell contains two parallel platinum electrodes coated with platinum black, each with a surface area S, and separated from the other by a distance L. The electrodes are protected by a glass tube. Other types of cells may also be used.Procedure
Determination of the cell constant. Use a certified reference material (such as a solution of potassium chloride) with conductivity less than 1500 S cml and the cell constant shall be within 2 per cent of the given value. A high cell constant is necessary when solutions of high conductivity are tested.Commonly used conductivity cells have cell constants of the order of 0.1 cm-1, 1 cm-1 and 10 cm-1.
Use a certified reference material (such as a solution of potassium chloride) with a conductivity value near the expected value of the solution under examination. Rinse the cell several times with distilled water and at least twice with the certified reference material used for the determination of the cell constant of the conductivity cell. Measure the resistance of the conductivity cell using the certified reference material at 25 ± 1°.The cell constant is given by the expression
 K = RemX Kern,where, Remis the measured resistance in megaohms and Kernis the conductivity of the certified reference material solution used, in microsiemens per centimetre.
K = RemX Kern,where, Remis the measured resistance in megaohms and Kernis the conductivity of the certified reference material solution used, in microsiemens per centimetre.NOTE - Other certified reference materials may be used especially for cells having a constant of 0.1 cm -1.
The measured constant K of the conductivity cell must be within 5 percent of the value indicated. If the cell constant is determined at a temperature other than that indicated for the certified reference material, the conductivity value is estimated from the expression:
Calibration of meter
Calibration can be done by replacing the conductivity cell with officially certified precision resistors (accurate to ± 0.1 percent of the stated value) or an equally accurate adjustable resistance device to give a predicted instrument response. Each scale on the meter may have to be calibrated prior to use. The instrument must have a minimum resolution of 0.1 ~ em-1.Related: SOP for Calibration of Conductivity Meter
Method
 After the apparatus has been calibrated with a certified reference material solution, rinse the conductivity cell several times with distilled water and at least twice with the aqueous solution under examination. Carry out successive measurements as described in the individual monograph.
After the apparatus has been calibrated with a certified reference material solution, rinse the conductivity cell several times with distilled water and at least twice with the aqueous solution under examination. Carry out successive measurements as described in the individual monograph.Water conductivity. A three-stage method of testing is described for Purified Water and Water for Injections (WFI). Testing at the first stage is usually adequate for Purified Water. Testing at two preliminary stages is given for WFI. If the test conditions and conductivity limits are met at either of these preliminary stages, the water meets the requirements of the test. In such cases proceeding to the third stage may not be necessary. Only in the event of failure at the final stage is the sample to be considered as not complying with the requirements of the test.
Stage 1 of the procedure may alternatively be performed online (with suitable modifications of the first step) with instrumentation that has been appropriately calibrated, whose cell constants have been accurately determined, and whose temperature compensation has been disabled. Prior to testing it must be ensured that such instrumentation has been suitably located and fitted in the water system.

Procedure
Stage 1
1. Measure the temperature of the water using a nontemperature-compensated conductivity reading. The measurement may be done in a suitable container or as an on-line determination.2. Using Table 1 find the temperature value that is not greater than the measured temperature and read the corresponding conductivity value that becomes the limit (Do not interpolate).
3. If the measured conductivity is not greater than the table value, the water meets the requirements of the test. If the conductivity is higher than the table value, proceed with testing at stage 2.
Stage 2
4. Transfer a sufficient amount of water (100 ml or more) to a suitable container, and stir the test sample. Adjust the temperature, if necessary, and while maintaining it at 25 ± 1°, begin vigorously agitating the sample while periodically observing the conductivity. When the change in conductivity (due to uptake of atmospheric carbon dioxide)is less than 0.1 ~ cm-1 per 5 minutes, note the conductivity.5. If the conductivity is not greater than 2.1 ~.cm-1, the water under examination meets the requirements of the test. If the conductivity is greater than 2.1 ~ cm-1, proceed with the testing at stage 3.
Stage 3
6. Perform this test within approximately 5 minutes of the conductivity determination in step 5 under stage 2, while maintaining the sample temperature at 25 ± 1°. Add a recently prepared saturated solution of potassium chloride to the test sample (0.3 ml per 100 ml of the test sample), and determine the pH to the nearest 0.1 pH unit.7. Using Table 2, determine the conductivity limit at the measured pH value in step 6. If the measured conductivity in step 4 under stage 2 is not greater than the conductivity requirements for the pH determined, the water under examination meets the requirements of the test. If either the measured conductivity is greater than this value or the pH is outside the range of 5.0 to 7.0, the water under examination does not meet the requirements of the test.
Also see: Validation of Purified Water System



No comments:
Post a Comment
Please don't spam. Comments having links would not be published.