1.0 OBJECTIVE
To lay down a procedure is to provide guidelines for Bacterial Endotoxin Test.2.0 SCOPE
This procedure is applicable for Bacterial Endotoxin Test.3.0 RESPONSIBILITY
Microbiologist4.0 ACCOUNTABILITY
Head QA & QC5.0 PROCEDURE
5.1 General
5.1.1 Clean all the required glassware’s and depyrogenate as per SOP for depyrogenation of glassware for BET.5.1.2 Transfer all the pyrogen free glassware to BET Room.
5.1.3 Switch “ON” the heating block as per SOP of operating Instruction for Heating Block and set the temperature at 37°C
5.1.4 Calculate MVD for sample specimen by the following formula -
5.1.5 Dilute the sample to be tested to its one-fourth MVD with LRW and shake on a vortex shaker for 3 minutes.
5.1.6 Sampling & Testing for Water for Injection: Collect the Water for injection sample in depyrogenated vials or test tubes as per sampling plan. Testing of water for injection is to be done as per the method of analysis.
5.2 Preparation of Control Standard (CSE)
5.2.1 Reconstitute CSE in LRW as per manufacturer's instruction as laid down in the bottle and certificate supplied by manufacturer, and vortex for 30 minutes intermittently.5.2.2 Use reconstituted CSE within 4 weeks after reconstitutions.
5.2.3 Prepare a CSE dilution with LRW to yield 1EU/ml and shake the dilution on vortex for 3 minutes.
5.2.4 Prepare a 4λ by diluting a volume, which is equivalent to 4λ concentration from 1 EU/ml to LRW. Shake the dilution on vortex for 3 minutes. (4λ to be back-calculated from l concentration. i.e. λ is the Lysate sensitivity).
5.3 Reconstitution of Lysate
5.3.1 Now reconstitute the lysate by opening the aluminum seal of lysate with the help of sterile blade.5.3.2 Collect lysate powder into the bottom of the vial by tapping on a hard surface. And then open the cork slowly.
5.3.3 As per manufacturer’s instruction, add LRW or buffer solution before use and replace the cork immediately.
5.4 Confirmation of the Labeled Lysate Sensitivity
5.4.1 Confirm in four replicates the labeled sensitivity λ expressed in EU/ml or IU/ml, of the lysate solution prior to use in the test. Confirmation of the lysate sensitivity must be carried out when a new batch of lysate is used or when there is any change in the experimental conditions which may affect the outcome of the test.5.4.2 Prepare CSE dilutions from 4λ solutions of at least four concentrations equivalent to 2λ, λ 0.5λ and 0.25λ by diluting a series of CSE solution with water for BET (LRW).
5.4.3 Take 10 depyrogenated assay tubes and label the tubes by numbering and arrange quadruplicate in the stand and then proceed as per the following table -
5.4.4 Pipette 100 ml diluted CSE i.e. to 2λ, λ, 0.5λ, and 0.25λ separately into depyrogenated test tubes (10 mm x75 mm) and labeled accordingly. For NWC (negative water control) use 100 ml LRW separately and perform the test in quadruplicate.
5.4.5 Add 100 ml of reconstituted Lysate into each tube and mix gently.
5.4.6 Incubate the reaction mixture for a constant period at 37° ± 1° for 60 ± 2 minutes, avoiding vibration.
5.4.7 After incubation, take each tube and turn directly from the incubator and invert it through approximately 180° in one smooth motion. If a firm gel has formed that remains in place upon inversion, record the result as positive. A result is negative if an intact gel is not formed.
5.4.8 The test is not valid unless the lowest concentration of the standard solutions shows a negative result in all replicate tests.
5.4.9 The endpoint is the last positive result in the series of decreasing concentrations of endotoxin. Calculate the mean value of the logarithms of the end-point concentrations and then the antilogarithm of the mean value using the following expression:
5.4.10 The geometric mean end-point concentration is the measured sensitivity of the lysate solution (EU/ml or IU/ml). If this is not less than 0.5λ and not more than 2λ, the labeled sensitivity is confirmed and is used in the tests performed with this Lysate.
5.5 Test Procedure
5.5.1 Prepare solutions A, B, C and D as shown in Table 1,Table – 1
5.5.2 Take 8 depyrogenated assay tubes and label the tubes by numbering and arrange in stand one opposite to each other. i.e. 1 & 2 for NPC, 3 & 4 for PPC, 5 & 6 for PWC, 7 & 8 for Negative water control.
5.5.3 Add 50 ml of LRW in NPC and PWC, and 100 ml in NWC. Immediately add 50 ml of the product sample, which is diluted at MVD/4 in a NPC and PPC, and then add 50ml of CSE that is diluted to 4λ in a PPC and PWC.
5.5.4 Finally add 100 ml of Lysate in all tubes and next, mix the assay tubes by hand and incubate in the heating block, where the temperature is maintained at 37 ± 1°C for 60 ± 2 minutes.
5.6 Interpretation of Result
5.6.1 Each tube in the gel clot method is interpreted as either positive or negative, the Positive test indicates the formation of firm gel capable of maintaining its integrity when the test tube is inverted 180°.5.6.2 A negative test is characterized by the absence of gel or by the formation of a viscous mass, which does not hold when the tube is inverted at 180°.
5.6.3 The test is not valid unless both replicates of the two positive control solutions B and C are positive and those of the negative control solution D are negative.
5.6.4 The preparation being examined complies with the test when a negative result is found for both replicates of solution A.
5.6.5 When a positive result is found for both replicates of solution A, it does not comply with the test.
5.6.6 Repeat the test if a positive result is found for one replicate of solution A and a negative result is found for the other. The preparation being examined complies with the test if a negative result is found for both replicates of solution A in the repeat test.
5.7 Failure Investigation
5.7.1 When positive result observed on both the tubes of test preparation, investigate the cause of its failure by checking following parameters.5.7.2 Check product dilution, CSE dilution and lysate dilution.
5.7.3 Whether Glassware are cleaned and depydrogenated as per SOP for cleaning of glassware.
5.7.4 Check sensitivity report of Lysate lot and matched CSE.
5.7.5 Check Heating block temperature and calibration.
5.7.6 Check Micropipette calibration.
5.7.7 Check any source of contamination occurs due to microbiologist.
5.8 Precaution
5.8.1 Rehydrated CSE may be stored for 28 days at 2 to 8°C.5.8.2 The supplied COA is specific for lysate lot and CSE lot. So use the same lot for testing
5.8.3 Do not vortex lysate.
5.9 Frequencies
5.9.1 Confirmation of Lysate sensitivity – Each lot of consignment.5.9.2 As per sampling plan for Water for Injection.
5.9.3 Batch wise for Finished Product and Raw Material.
6.0 ABBREVIATIONS
SOP: Standard Operating ProcedureQA: Quality Assurance
QC: Quality Control
NA: Not Applicable
BET: Bacterial Endotoxin Test
CSE: Control Standard Endotoxin
MVD: Maximum Valid Dilution
LRW: Lal Reagent Water
PPC: Positive Product Control
NPC: Negative Product Control
PWC: Positive Water Control
NWC: Negative water control
Get ready to use editable SOPs in MS-Word FormatView List

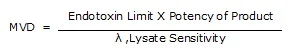





No comments:
Post a Comment
Please don't spam. Comments having links would not be published.