This method for determining total organic carbon (TOC) indirectly measures the total amount of organic substances present in water for pharmaceutical use. The molecules of organic matter in water are oxidized to produce carbon dioxide which is then measured in an instrument and from the result, the concentration of carbon in the water is calculated.
The determination of carbon in water may be made either online (in the line of supply of the water) or offline. Irrespective of the method used, the system is qualified by analyzing a standard solution of a substance that is easily oxidizable (such as sucrose) at a concentration adjusted to give an instrument response corresponding to the TOC limit to be measured, and by interpreting the results in limit tests.
The determination of carbon in water may be made either online (in the line of supply of the water) or offline. Irrespective of the method used, the system is qualified by analyzing a standard solution of a substance that is easily oxidizable (such as sucrose) at a concentration adjusted to give an instrument response corresponding to the TOC limit to be measured, and by interpreting the results in limit tests.
The suitability of the system is determined by analysis of a solution prepared with a substance that is oxidizable with difficulty (such as l,4-benzoquinone).
Apparatus:
Any suitable apparatus capable of discriminating between organic and inorganic carbon either by purging inorganic carbon from the sample under examination before oxidization or by the measurement of the inorganic carbon and subtraction from the total carbon may be used.
The instrument manufacturer's instructions should be followed for installation and subsequent operations. The instrument should be calibrated and the system suitability should be verified at suitable intervals. The apparatus must have a limit of detection specified by the manufacturer of 0.05 mg or less of carbon per litre.
Glassware: Use glassware that has been thoroughly cleaned by a method that will remove organic matter. Use TOC water for the final rinse of glassware.
Solutions
TOC water:
Highly purified water complying with the following specifications:
Conductivity:
Not more than 1.0 microS cm-1 at 25°.
Test solution:
Collect carefully the water to be tested in an airtight container with minimum head space and examine it with minimum delay.
Standard solution:
Dissolve sucrose, previously dried at 105° for 3 hours, in sufficient TOC water to produce a solution containing 1.19 mg of sucrose per litre (0.50 mg of carbon per liter).
System suitability solution:
Dissolve 1,4-benzoquinone in sufficient TOC water to produce a solution containing 0.75 mg of 1,4-benzoquinone per liter (0.50 mg of carbon per liter).
NOTE - Use TOC water obtained at the same time as that used to prepare the standard solution and the system suitability solution.
Control solutions:
Prepare suitable blank solutions or other solutions needed for establishing the base for calibration adjustments. Run the appropriate blanks for zeroing the instrument.
System suitability:
Run successively the TOC water, standard solution and system suitability solution and record the responses rw, rs and rs" respectively. Calculate the percentage response efficiency from the expression:
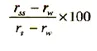
The system is suitable if the response efficiency is not less than 85 per cent and not more than 115 percent of the theoretical response.
Procedure:
Run the test solution and record the response, rt. The test solution complies with the test if rt is not greater than rs-rw.




No comments:
Post a Comment
Please don't spam. Comments having links would not be published.