This test is designed to determine compliance with the dissolution requirements for solid dosage forms administered orally. The test is intended for a capsule or tablet.
Use Apparatus I unless otherwise directed. All parts of the apparatus that may come into contact with the preparation under examination or with the dissolution medium are chemically inert and do not absorb, react or interfere with the preparation under examination. All metal parts of the apparatus that may come into contact with the preparation or the dissolution medium must be made from stainless steel, type 316 or equivalent or coated with a suitable material to ensure that such parts do not react or interfere with the preparation under examination or the dissolution medium. No part of the assembly, including the environment in which the assembly is placed, contributes significant motion, agitation or vibration beyond that due to the smoothly rotating element.

a. A cylindrical vessel, A, made of borosilicate glass or any other suitable transparent material, with a hemispherical bottom and with a nominal capacity of 1000 ml and an inside diameter of 98-106 mm (Fig.). The vessel has a flanged upper rim and is fitted with a lid that has a number of openings, one of which is central.
b. A motor with a speed regulator capable of maintaining the speed of rotation of the paddle within 4 percent of that specified in the individual monograph. The motor is fitted with a stirring element which consists of a drive shaft and blade forming a paddle, B (Fig.). The blade passes through the diameter of the shaft so that the bottom of the blade is flush with the bottom of the shaft. The shaft is positioned so that its axis is within 2 mm of the axis of the vessel and the lower edge of the blade is 23 to 27 mm from the inside bottom of the vessel. The apparatus operates in such a way that the paddle rotates smoothly and without significant wobble.
c. A water-bath set to maintain the dissolution medium at 36.5° to 37.5°. The bath liquid is kept in constant and smooth motion during the test. The vessel is securely clamped in the water bath in such a way that the displacement vibration from other equipment, including the water circulation device, is minimized.
The assembly is the same as in Apparatus 1 except that in the stirring element the paddle is replaced by a basket, D (see Figs.). The metallic shaft rotates smoothly and without significant wobble. The basket consists of two components. The top part, with a vent, is attached to the shaft C, it is fitted with three spring clips, or other suitable means, that allow removal of the lower part for introduction of the preparation under examination and that firmly hold the lower part of the basket concentric with the axis of the vessel during rotation. The lower detachable part of the basket is made of welded-steam cloth, with a wire thickness of 0.254 mm diameter and with 0.381 mm square openings, formed into a cylinder with a narrow rim of sheet metal around the top and the bottom. The basket may be plated with a 2.5 mm layer of gold for use with acidic media. The distance between the inside bottom of the vessel and the basket is maintained at 23 to 27 mm during the test.
Operate the apparatus immediately at the speed of rotation specified in the individual monograph. Within the time interval specified, or at each of the times stated, withdraw a specimen from a zone midway between the surface of the dissolution medium and the top of the rotating blade or basket, not less than 10 mm from the wall of the vessel. Except in the case of single sampling, add a volume of dissolution medium equal to the volume of the samples withdrawn. Filter the sample solution promptly through a membrane filter disc with an average pore diameter not greater than 1.0 micron. Discard the first few ml of the filtrate. Perform the analysis as directed in the individual monograph. Repeat the whole operation five times. Where two or more tablets or capsules are directed to be placed together in the apparatus, carry out six replicate tests.
For each of the tablet or capsule tested, calculate the amount of dissolved active ingredient in solution as a percentage of the stated amount where two or more tablets or capsules are placed together, determine for each test the amount of active ingredient in solution per tablet or capsules and calculate as a percentage of the stated amount.
Correction factors should not be greater than 25 percent of the stated amount.
Modified-release dosage forms. Use method A or Method B.
Related: Tablet Dissolution Test in Different Stages (S1, S2 and S3)

Related: Calibration of Dissolution Testing Apparatus
Use Apparatus I unless otherwise directed. All parts of the apparatus that may come into contact with the preparation under examination or with the dissolution medium are chemically inert and do not absorb, react or interfere with the preparation under examination. All metal parts of the apparatus that may come into contact with the preparation or the dissolution medium must be made from stainless steel, type 316 or equivalent or coated with a suitable material to ensure that such parts do not react or interfere with the preparation under examination or the dissolution medium. No part of the assembly, including the environment in which the assembly is placed, contributes significant motion, agitation or vibration beyond that due to the smoothly rotating element.

Apparatus 1
An assembly consisting of the following:a. A cylindrical vessel, A, made of borosilicate glass or any other suitable transparent material, with a hemispherical bottom and with a nominal capacity of 1000 ml and an inside diameter of 98-106 mm (Fig.). The vessel has a flanged upper rim and is fitted with a lid that has a number of openings, one of which is central.
b. A motor with a speed regulator capable of maintaining the speed of rotation of the paddle within 4 percent of that specified in the individual monograph. The motor is fitted with a stirring element which consists of a drive shaft and blade forming a paddle, B (Fig.). The blade passes through the diameter of the shaft so that the bottom of the blade is flush with the bottom of the shaft. The shaft is positioned so that its axis is within 2 mm of the axis of the vessel and the lower edge of the blade is 23 to 27 mm from the inside bottom of the vessel. The apparatus operates in such a way that the paddle rotates smoothly and without significant wobble.
c. A water-bath set to maintain the dissolution medium at 36.5° to 37.5°. The bath liquid is kept in constant and smooth motion during the test. The vessel is securely clamped in the water bath in such a way that the displacement vibration from other equipment, including the water circulation device, is minimized.
Dissolution medium
Use the dissolution medium specified in the individual monograph. If the medium is a buffered solution, adjust the solution so that its pH is within 0.05 units of the pH specified in the monograph. The dissolution medium should be deaerated prior to testing.Time
Where a single time specification is given in the monograph, the test may be concluded in a shorter period if the requirement for the minimum amount dissolved is met. If two or more times are specified, the specimen is to be withdrawn only at the stated times, within a tolerance of ± 2 percent.Method
Conventional and prolonged-release solid dosage forms
Place the stated volume of the dissolution medium, free from dissolved air, into the vessel of the apparatus. Assemble the apparatus and warm the dissolution medium to 36.5° to 37.5°. Unless otherwise stated, place one dosage unit in the apparatus, taking care to exclude air bubbles from the surface of the dosage unit. When Apparatus 1 is used, allow the tablet or capsule to sink to the bottom of the vessel prior to the rotation of the paddle. A suitable device such as a wire of glass helix may be used to keep horizontal at the bottom of the vessel tablets or capsules that would otherwise float. When Apparatus 2 is used, place the tablet or capsule in a dry basket at the beginning of each test. Lower the basket into position before rotation.Operate the apparatus immediately at the speed of rotation specified in the individual monograph. Within the time interval specified, or at each of the times stated, withdraw a specimen from a zone midway between the surface of the dissolution medium and the top of the rotating blade or basket, not less than 10 mm from the wall of the vessel. Except in the case of single sampling, add a volume of dissolution medium equal to the volume of the samples withdrawn. Filter the sample solution promptly through a membrane filter disc with an average pore diameter not greater than 1.0 micron. Discard the first few ml of the filtrate. Perform the analysis as directed in the individual monograph. Repeat the whole operation five times. Where two or more tablets or capsules are directed to be placed together in the apparatus, carry out six replicate tests.
For each of the tablet or capsule tested, calculate the amount of dissolved active ingredient in solution as a percentage of the stated amount where two or more tablets or capsules are placed together, determine for each test the amount of active ingredient in solution per tablet or capsules and calculate as a percentage of the stated amount.

Acceptance criteria
Conventional-release dosage forms
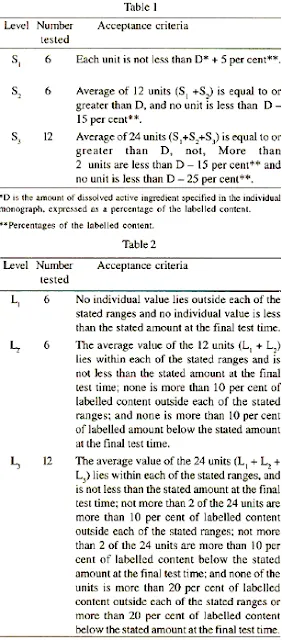
Unless otherwise specified, the requirements are met if the quantities of active substance dissolved from the dosage units conform to Table 1. If the results do not conform to the requirements at stage S) given in the table, continue testing with additional dosage units through stages S2 and S3 unless the results confirm at stage S2' Where capsule shells interfere with the analysis, remove the contents of not less than 6 capsules as completely as possible, and dissolve the empty capsule shells in the specified volume of the dissolution medium. Perform the analysis as directed in the individual monograph. Make any necessary correction.Correction factors should not be greater than 25 percent of the stated amount.
Prolonged-release dosage forms
Unless otherwise specified, the requirements are met if the quantities of active substance dissolved from the dosage units conform to Table 2. If the results do not conform to the requirements at stage L1 given in the table, continue testing with additional dosage units through stages L2 and L1 unless the results conform at stage L2. The limits embrace each value of D, the amount dissolved at each specified dosing interval. Where more than one range is specified, the acceptance criteria apply to each range.Modified-release dosage forms. Use method A or Method B.
Method A
Acid stage. Place 750 ml of a.1M hydrochloric acid in the vessel, and assemble the apparatus. Warm the dissolution medium to 36.5° to 37.5°. Place one dosage unit in the apparatus, cover the vessel and operate the apparatus at the specified rate. After 2 hours of operation in the acid medium, withdraw an aliquot of the liquid and proceed immediately as directed under Buffer stage. Perform the analysis of the aliquot using a suitable assay method.Buffer stage
Complete the operations of adding the buffer and adjusting the pH within 5 minutes. With the apparatus operating at the rate specified, add to the medium in the vessel 250 ml of a 0.2 M solution of trisodium phosphate dodecahydrate that has been warmed to 36° to 37°. Adjust, if necessary, with 2M hydrochloric acid or 2 M sodium hydroxide to a pH of 6.8 ± 0.05. 2 M hydrochloric acid or 2 M sodium hydroxide to a pH of 6.8 ± 0.05.Related: Tablet Dissolution Test in Different Stages (S1, S2 and S3)
Method B
Acid stage. Place 1000 ml of 0.1M hydrochloric acid in the vessel and assemble the apparatus. Warm the dissolution medium to 36° to 37°. Place one dosage unit in the apparatus, cover the vessel and operate the apparatus at the specified rate. After 2 hours of operation in the acid medium, withdraw an aliquot of the liquid and proceed immediately as directed under Buffer stage. Perform the analysis of the aliquot using a suitable assay method.
Buffer stage
Use a buffer that has previously been warmed to 36°to 37°. Drain the acid from the vessel and add 1000 ml of pH 6.8 phosphate buffer, prepared by mixing 3 volumes of 0.1M hydrochloric acid with 1 volume of 0.2 M solution of trisodium phosphate dodecahydrate and adjusting, if necessary, with 2M hydrochloric acid or 2M sodium hydroxide to a pH of 6.8 ± 0.05. This may also be done by removing from the apparatus the vessel containing the acid and replacing it with another vessel containing the buffer and transferring the dosage unit to the vessel containing the buffer. Continue to operate the apparatus for 45 minutes, or for the specified time. At the end of this period, withdraw an aliquot of the liquid and perform the analysis using a suitable assay method.Acceptance criteria
Acid stage
Unless otherwise specified, the requirements of this part of the test are met if the quantities, based on the percentage of the labeled content of active substance dissolved from the units tested conform to Table 3. Continue the testing through the 3 levels unless the results of both acid and buffer stages conform at an earlier level.Related: Calibration of Dissolution Testing Apparatus





good information
ReplyDelete