There are two major categories of solid phases depending on the molecular packaging order. This is the most common crystalline state that is characterized by both short-term and long-term order, meaning that a consistent structure reaches throughout the entire crystal. An amorphous solid, on the other hand, has a regular structure that is limited to the near neighbors of the molecule at the center of the solid. In terms of solid crystals, polymorphs can be divided into monomorphic crystals and polymorphic crystals, as well as multi-component crystals such as hydrates, solvates, and cocrystals, composed of more than one molecule type.

Crystalline solid
Molecular and atomic arrays held together by non-covalent interactions make up crystals. An inorganic salt, sodium chloride, is a simple example of a unit cell. It can be seen in Figure 1 that the ions are arranged in an orderly manner within the crystal structure of sodium chloride. The chloride ions can be connected with a square on one side. It is possible to draw squares on all sides of a cube to form a unit cell, a repeating cubic unit. Molecular ions or molecules arranged in the same way are contained in the same size and arrangement within each unit cell within a specific crystal. Considering atoms or molecules, it tends to be easier to consider crystals as a pattern of three-dimensional points. Figure 1
There are seven primitive unit cells for all possible crystals, as shown in Figure 2. A primitive unit cell is illustrated in figure 2 by showing the lengths and angles of its sides. a, b, and c are the lengths of each side, while a, b, and g represent the angles between them. There are atoms or molecules present at the corners of each unit cell in the structures in Fig., 2. A unit cell can also include atoms or molecules centered at the ends or bottom (end-centered), centered at every face (face-centered), or centered at the center itself (body-centered), as in Fig. 3. In the pharmaceutical industry, triclinic, monoclinic, and orthorhombic cells are the three most common types of unit cells.
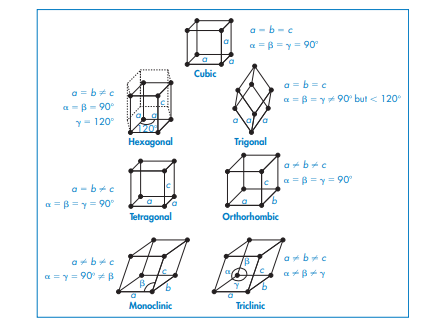
There are seven primitive unit cells for all possible crystals, as shown in Figure 2. A primitive unit cell is illustrated in figure 2 by showing the lengths and angles of its sides. a, b, and c are the lengths of each side, while a, b, and g represent the angles between them. There are atoms or molecules present at the corners of each unit cell in the structures in Fig., 2. A unit cell can also include atoms or molecules centered at the ends or bottom (end-centered), centered at every face (face-centered), or centered at the center itself (body-centered), as in Fig. 3. In the pharmaceutical industry, triclinic, monoclinic, and orthorhombic cells are the three most common types of unit cells.
Figure 2
We find that these variations do not apply to every type of unit cell:

We find that these variations do not apply to every type of unit cell:
- Body-centred, cubic, tetragonal and orthorhombic
- End-centered monoclinic and orthorhombic
- Face-centered cubic and orthorhombic
Figure 3
We call these the Bravais lattices because there are 14 kinds of unit cells in all.
However, because it is difficult to arrange large molecules in an orderly fashion, molecules of this size usually do not form perfect crystals. Material of this type is normally semicrystalline, with amorphous zones surrounding crystalline regions, as shown in Figure 4. The glass transition temperature Tg changes the physical properties of semicrystalline or amorphous materials. There is no sharp melting point in amorphous or semicrystalline materials. Temperatures above Tg cause the molecules to become mobile, resulting in rubbery materials. A glassy state is present below Tg, and the material is brittle. Generally, plasticizers are small molecules that fit between glassy molecules, lowering the transition temperature. This increases the mobility of glassy molecules.

We call these the Bravais lattices because there are 14 kinds of unit cells in all.
Amorphous solid
An amorphous solid consists of molecules that do not follow any long-range order. The disordered structures provide alternatives to their crystalline counterparts in drug delivery formulations due to their solubility, stability, dissolution, and compressibility properties. It is theoretically possible to prepare many classes of materials in their amorphous state by solidifying them at a higher rate than their molecules can organize themselves into three-dimensional crystal structures. The crystalline material is susceptible to inadvertent conversion to amorphous by mechanical or thermal energy, such as when it is ground, compressed, or milled, or when it is dried. Certain materials are inherently amorphous, such as polyethylene glycol, polyvinylpyrrolidone, and poly (lactic acid).However, because it is difficult to arrange large molecules in an orderly fashion, molecules of this size usually do not form perfect crystals. Material of this type is normally semicrystalline, with amorphous zones surrounding crystalline regions, as shown in Figure 4. The glass transition temperature Tg changes the physical properties of semicrystalline or amorphous materials. There is no sharp melting point in amorphous or semicrystalline materials. Temperatures above Tg cause the molecules to become mobile, resulting in rubbery materials. A glassy state is present below Tg, and the material is brittle. Generally, plasticizers are small molecules that fit between glassy molecules, lowering the transition temperature. This increases the mobility of glassy molecules.
Figure 4
Film coatings use a wide range of polymers, including water, as plasticizers. Because amorphous solids are less stable and exhibit a higher energy state, they can eventually convert into crystalline solids, which are more stable thermodynamically. Furthermore, due to their higher molecular mobility, they often have stronger chemical reactivity and, therefore, a higher rate of chemical degradation. Nevertheless, amorphous forms of drugs often have a higher solubility than crystalline forms and this may provide an opportunity to enhance their bioavailability for poorly water-soluble drugs.

Figure 5Film coatings use a wide range of polymers, including water, as plasticizers. Because amorphous solids are less stable and exhibit a higher energy state, they can eventually convert into crystalline solids, which are more stable thermodynamically. Furthermore, due to their higher molecular mobility, they often have stronger chemical reactivity and, therefore, a higher rate of chemical degradation. Nevertheless, amorphous forms of drugs often have a higher solubility than crystalline forms and this may provide an opportunity to enhance their bioavailability for poorly water-soluble drugs.
Polymorphism
The crystallization habits of compounds depend on the conditions of crystallization. It is typical for crystal habits to share the same internal structure, which is also reflected in their diffraction patterns. The properties of the compounds may differ more fundamentally when they crystallize as polymorphs. Polymorphism can manifest itself as differences in molecule orientation or position at the lattice sites; either the molecules can arrange themselves differently within the crystal lattice or the molecules can manifest themselves differently in the crystal. Different variations result in different polymorph X-ray diffraction patterns, and this technique can be used to detect polymorphs. A polymorph may have very different properties in both physical and chemical terms. For example, its melting point and solubility may be distinctly different. They may also exist in distinct habits. Let us consider two examples of polymorphs. Spironolactone (I) crystallizes in two polymorphic forms, four solvated polymorphic forms, and two solvated crystalline forms depending upon the solvent and method used for crystallization.Polymorphism in pharmacy
Different crystal habits can cause problems with tableting and injection. They too will be affected by these problems since polymorphs have frequently different habits. Polymorphs, however, are likely to have different crystal lattices, and their energy contents will be significantly different from one another, as a result of which their stability and bio-pharmacological activity will be different. As a result of a different arrangement of molecules or ions, the different polymorphs in the solid-state will have different interactions energies. The polymorphic form exhibiting the lowest free energy is the most stable and the rest is likely to transform into it when the conditions are right. To convert two polymorphs into one, the energy barrier between them must be large enough to offer enough energy - the greater the energy barrier and the lower the storage temperature, the slower the conversion rate.
Get subject wise printable pdf notesView Here



No comments:
Post a Comment
Please don't spam. Comments having links would not be published.